Integrating data into one platform: a better analysis of the cascade of care
Nigeria’s experience containing the Ebola epidemic of 2014 highlighted the critical need for a comprehensive data management system to deal with health outbreaks.
Using digital tools data science can strengthen disease management, as the country’s Covid-19 case study (FIND Digital Health Report, April 2021) shows.
This included integrating real-time surveillance and case management, aiming at more agile and efficient responses to future outbreaks.
Data in detail
Nigeria registered its first Covid-19 case on February 27, 2020.
Working closely with state health teams and partners, the Nigeria Centre for Disease Control (NCDC) led the response.
By March 29, 2021, more than 160 000 cases had been diagnosed from over 1.7-million tests.
Structure and data flow: SORMAS
The outbreak of Ebola resulted in the NCDC and partners developing Nigeria’s Surveillance, Outbreak Response Management and Analysis System (SORMAS).
This system expanded to support managing other priority diseases, including Covid-19, in January 2020, and was rapidly scaled up across Nigeria over the pandemic.
SORMAS is an end-to-end digital solution that captures geocoded data on all steps of the Test-Trace-Isolate cascade of Covid-19.
Using this tool, Nigeria has been able to capture data at multiple levels to guide policy and programme interventions.
By 2021 Nigeria had several additional patient-facing tools, including:
- The NCDC ChatBot accessed via the NCDC’s website
- The self-assessment web application developed by Wellvis with NCDC
- The Interactive Voice Response solution available as a call-back service
- The USSD self-assessment solution
- The Disease Control Hotlines established in many states.
Covid-19 screening, testing and management
The graphic below shows how the NCDC ChatBot and Wellvis channels feed data to the dashboard, and to SORMAS, in the areas of screening, testing and management.
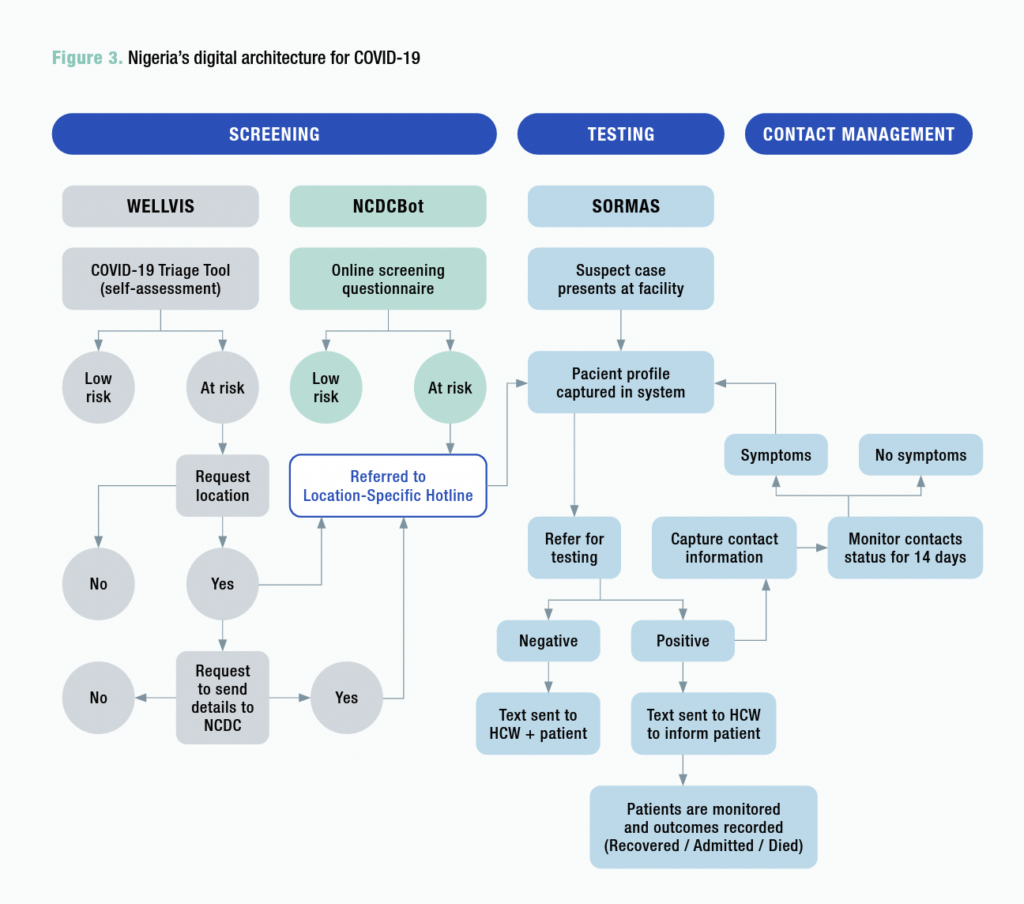
Why it works
- Different dashboards are tailored to users at various levels of the health system
- Data is transmitted to the NCDC website to provide to the public
- Built-in algorithms generate early warnings for outbreaks, and SORMAS also generates data for surveillance and epidemiological analysis.
BENEFITS: Nigeria’s digital tools
- Improvement in data quality
- Better decision-making: SORMAS ensures all key demographic, epidemiological and clinical variables are recorded
- Trigger warnings: users are notified of missing or incorrect key variables, preventing the next step until these are resolved
- Central platform: routine recording of all metrics (test-trace-isolate phases) helps avoid additional data collection
- Complete visibility: real-time supervision for health workers, motivating improved performance and guideline compliance.
Practical steps to develop scale
SORMAS was developed through a multi-institutional collaboration led by NCDC, the HZI, the African Field Epidemiology Network and German IT institutions. It transitioned from proprietary to open-source software in 2016 and its source code is accessible to software developers through the GitHub website.
In rolling out SORMAS, a training-of-trainers approach was used and, as new disease modules are added, this same approach is used.
Key success factors in Nigeria
- Strong national ownership of SORMAS from the start.
- As the NCDC actively promotes it as the preferred digital solution for Covid-19 management, there is less risk of multiple overlapping digital tools.
- Integration into the broader health system, as it pre-dates the pandemic and was already in use.
- “Design thinking” approach. SORMAS was responsive to the context in which the app was deployed, building on practical insights from the Ebola response.
- Can be used offline. Data stored on the platform is automatically uploaded with connectivity.
- Flexibility. A module-based approach means new ones can be rapidly added.
Operational challenges
One challenge is that mobile devices with old operating systems are unable to optimally support the SORMAS software.
Too few staff at implementation level can lead to delays in data being entered into SORMAS, negating the benefit of a real-time data capture system. However, the public should be able to input more data in the future, reducing the time taken by health care workers.
Key takeaways for other countries
SORMAS has led to improved data management and containment of the epidemic in Nigeria.
Ensuring automated data transmission between digital tools, and consolidating this using unique patient IDs, helps with comprehensive management of the pandemic.
Agility in digital tool development is vital, with continual changes to SORMAS to improve it.
Nigeria’s experience shows that integrating data into one platform enables better analysis of the cascade of care.
It also facilitates communication and referral between multiple players at different levels of the health system.





